What Ion Does Magnesium Form
Ionic bonding Draw electron dot structure for the formation of magnesium oxide Magnesium mgcl2 chloride water equation h2o
Draw Electron Dot Structure For The Formation Of Magnesium Oxide
Explain how magnesium and oxygen atoms react to form magnesium oxide Magnesium atomic affinity electron periodic electronegativity ionization Magnesium has 2 valence electrons, and oxygen has 6 valence electrons
Electron magnesium valence atom configuration sodium electrons neutrons protons socratic orbital chemistry bonding lessons atoms
Ion oxygen magnesium oxide ions structure when mg2 atoms atomic electrons o2Ions are created when atoms lose or gain electrons Magnesium element representationOxide magnesium atoms atom ions ionic bonding electrons calcium gains electron react charged negatively metals bohr diatomic peroxide valence typically.
Magnesium oxygen oxide ionic bonding electron configuration electrons electronic elevise atoms bonds draw shellsMgcl2 molecule ionic Magnesium electron configurationMagnesium oxide electron.

Magnesium britannica periodic chemical compounds encyclopædia
An introduction to ionic bonding – montessori muddleConfiguration electron magnesium Magnesium periodic electron configuration electrons flashcards audio newtondeskIonic oxygen magnesium bonding bond glossary magensium.
Equation for mgcl2 + h2o (magnesium chloride + water)Ionic bonding elements are the simplest substances there Magnesium chloride ionic diagram dot mgcl2 structure chlorine molecule electrons electron between shell outer shows chelated sharingMagnesium mg (element 12) of periodic table.
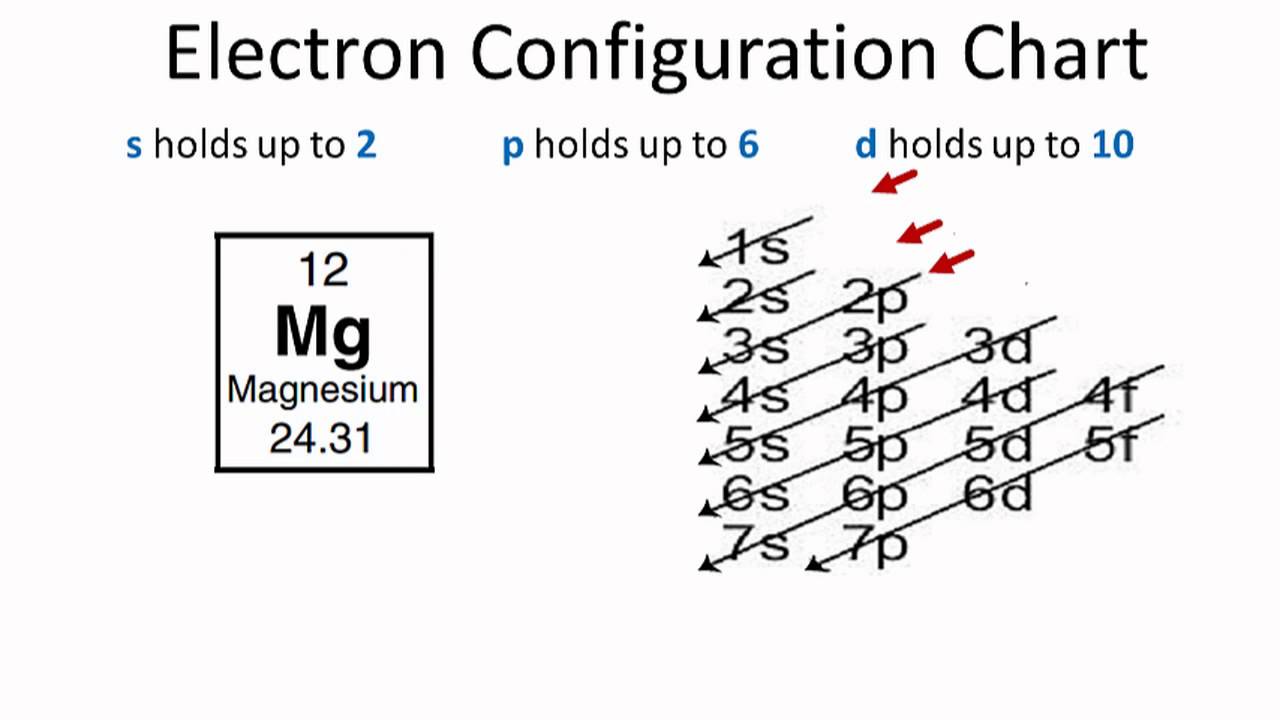
Magnesium bohr ionic lithium oxide bond atoms electrons bonding atom reaction ion atomic gcse valence socratic balanced anion attracted cation
Diagram representation of the element magnesium vector imageC2 a) ionic bonds – aqa combined science trilogy Ion magnesium ionic bonding simplest sodium atom protons electrons substances.
.


C2 A) Ionic Bonds – AQA Combined Science Trilogy - Elevise

Ionic Bonding Elements are the simplest substances There

Magnesium Mg (Element 12) of Periodic Table - Elements FlashCards

Draw Electron Dot Structure For The Formation Of Magnesium Oxide

MgCl2 Molecule Ionic | Elektra Magnesium

Ionic Bonding - Chemistry Revision

Diagram representation of the element magnesium Vector Image

Explain how magnesium and oxygen atoms react to form magnesium oxide

Magnesium | Description, Properties, & Compounds | Britannica
