Inert Electrode Electrolysis
4 inert electrodes Difference between active and inert electrodes Electrochemical cell conventions
Chapter 19.7: Electrolysis - Chemistry LibreTexts
Class xi-xii : electrolysis of aq. cuso4 using inert electrode Inert electrolysis edexcel chemistry electrodes Electrolysis copper sulphate inert electrodes using platinum graphite
Chapter 19.7: electrolysis
Electrolysis water acidified platinum electrodes inert icse acid sulphuric classElectrolysis electrodes inert reactive cuso4 Tutorke electrodes electrode electrolysis hydrogenElectrolysis of concentrated sodium chloride solution using graphite.
Cuso4 electrolysis using electrode aq inertCore practical 4.2 inert electrodes electrolysis gcse edexcel chemistry Electrolysis inert electrode chemistryCell electrode inert electrochemical anode voltaic example chemistry conventions cathode libretexts fe active cells cu.

Electrolysis of copper sulphate using inert electrodes
Inert electrodes voltaic cellsElectrolysis inert electrodes solution using solutions aqueous gif apparatus tubes originally filled small chemguide Igcse chemistry 2017: 1.58c: describe experiments to investigateElectrolysis electrodes inert ppt appropriate ions attract.
Ch. 20-3 inert electrodes/voltaic cellsElectrolysis of solutions with inert electrodes Electrolysis molten downs nacl electrolytic libretexts cl2Explain electrolysis of molten copper sulphate using inert electrodes.

Dilute electrolysis sodium chloride electrodes inert
Inert electrodes electrolysis active difference between copper chloride natural diagram electrode graphite example vs setup grade atoms decompose sciences figureElectrolysis of acidified water with platinum electrodes Inert electrodes active electrolysis chemistryStandard potentials.
Electrolysis sodium chloride solution concentrated electrodes using graphite nodeElectroylsis battery, electrodes and electrolyte (inert electrodes Electrode inert electroanalytical methods ppt powerpoint presentationElectrolysis bromide lead igcse ii aqueous solutions chemistry experiments diagram copper molten electrode inert using chloride acid sulfuric investigate dilute.

Copper electrolysis using molten sulphate electrodes inert explain dear student reactions equation occuring tell both also
Electrolysis gcse oxygen ions halideElectrolysis sulfate electrodes graphite equations Electrode inert electrodesStandard potentials potential electrode using chemistry hydrogen cell measuring determining.
Inert electrolyte electrodes(a) the set up below was used to investigate the products formed at the Chapter 19.7: electrolysisElectrolysis of dilute sodium chloride (inert electrodes).

Galvanic chemistry electrolysis electrolytic electrochemistry electrode anode cathode electrons reverse chem principles libretexts electron batteries corrosion
.
.

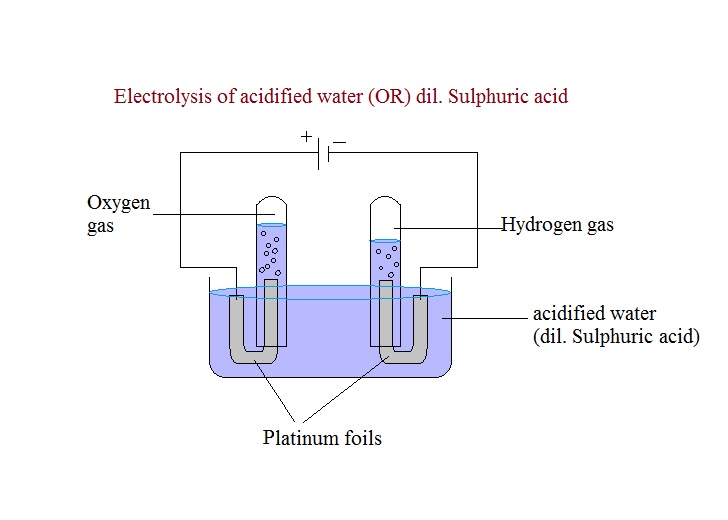
Electrolysis of acidified water with Platinum electrodes

Electrolysis | OCR Gateway C3 | revisechemistry.uk

PPT - Electroanalytical methods PowerPoint Presentation, free download

Chapter 19.7: Electrolysis - Chemistry LibreTexts

Electroylsis Battery, Electrodes and Electrolyte (inert electrodes

Electrolysis of dilute sodium chloride (inert electrodes) - YouTube

Explain electrolysis of molten copper sulphate using inert electrodes